The Translational Tissue Engineering Center (TTEC) is a hub for regenerative medicine—the branch of biomedical engineering that develops methods to regrow, repair or replace damaged cells, tissues and organs– and immunoengineering, which leverages design tools and technologies to study and manipulate the immune response. Our nine core faculty members span six departments at The Johns Hopkins University and include both clinicians and basic scientists.
Through The Discovery Of Fundamental Biological Principles, The Engineering Of New Technologies And The Clinical Testing Of Our Innovations, We Aim To Significantly Improve The Health Of Patients Suffering From An Array Of Maladies, Including Trauma, Cancer And Immune Disorders.
ILLUSTRATION BY TTEC AND MARTIN RIETVELD
Excellence
Our research projects broadly divide into two categories: tissue engineering and immunoengineering. Our recent discoveries in these areas put us in a unique position to take a national and international leadership position in this exciting field.
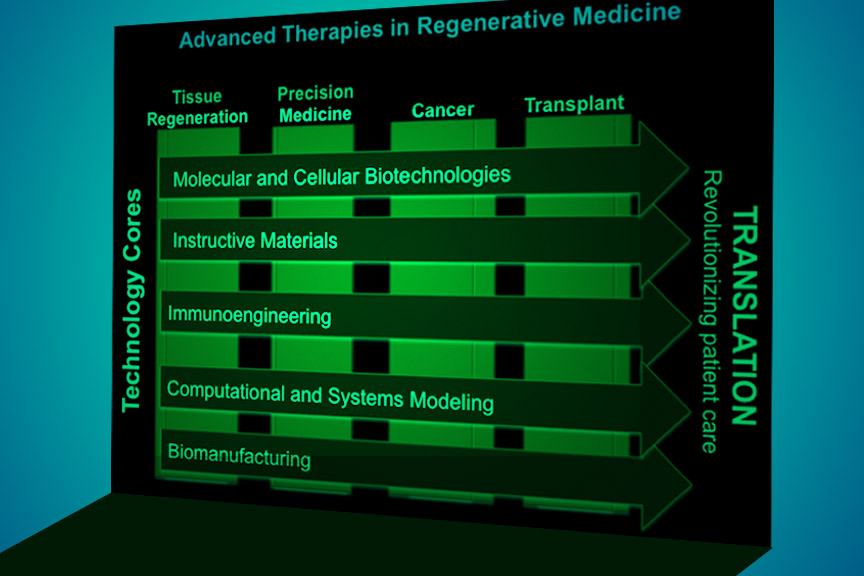
Below is an overview of our research strategy.
1. DISCOVERY
Discovery encompasses all elements of regenerative medicine: cells, materials, biological and developmental signals, and clinical practice.
Big data: Future breakthroughs will depend on rapid advances of technology for high-throughput generation—and interpretation—of experimental data at all scales.
Multiscale discovery, control, and systems modeling: Computational modeling is needed to integrate experimental data from one or more scales (e.g. the nanomolar scale) to predict biological behavior at larger scales (cell to tissue to whole organism scales).
Instructive materials: Detailed data about the physical, topographical, and biochemical cues that control cellular responses will be used to develop next-generation biomaterials to program stem cells and host tissues and to effectively target nanotherapeutics to diseased tissues and cells.
2. INNOVATION
Bridging discovery and translation, engineers are incorporating the latest discoveries into their design of technologies that solve today’s most challenging clinical problems.
Therapeutic molecular and cellular biotechnologies: To engineer cell function ex vivo and in vivo, current techniques include the design, delivery, and presentation of biological agents including DNA, siRNA, mRNA, miRNA, peptides, proteins, sugars, and small molecules.
Biomanufacturing: Ongoing and future efforts will center on clinical grade bioproduction capabilities including GMP facilities, organ farming and cost-effective “lab-on-a-chip” technologies.
Immunoengineering: We are developing platforms to modulate innate and adaptive immune responses to promote tissue regeneration and wound healing, control the fate and trafficking of drug delivery systems, potentiate vaccines, and transform cancer treatment options, and design novel treatments for autoimmune disorders.
3. TRANSLATION
The translation, or clinical implementation, of discoveries is key to improving patient care and understanding the primary therapeutic action of new technologies.
Regeneration of tissues: Engineers and clinical faculty must work closely together to design and translate to the clinic new therapies that provide functional tissue replacements for those lost due to trauma, disease or congenital abnormalities.
Cancer therapy: The connection between regeneration and cancer brings together diverse faculty and provides new and fruitful avenues of research, from targeted nanomedicines that destroy cancer cells but spare healthy ones to the manipulation of cancer sugar metabolism.
Precision medicine: Advanced delivery technologies will be combined with customized molecular targeting of specific inherited and immunologic diseases to develop superior therapies that account for an individual’s genetic composition, environment, and life-history.
Transplantation: New discoveries are emerging in the development of natural and synthetic biomaterials, bioreactors, and cellular technologies that improve the graft-host integration and functionality of post-transplant donor tissues and organs.
Autoimmune diseases: Many of the immunoengineering strategies developed for transplant tolerance will also be relevant to autoimmune diseases.
