Johns Hopkins engineers have helped develop and characterize an artificial protein that triggers the same response in the human body as its natural counterpart—a breakthrough that not only has the potential to facilitate the design of drugs to accelerate healing but also sheds light on the mechanisms behind various diseases.
‘Tipping the Balance’ of Immune Cells From Bad to Good Reverses Multiple Sclerosis Symptoms in Mice
Johns Hopkins Medicine team suggests that microparticle-delivered therapy may be first step toward stopping MS and other autoimmune diseases.
According to the federal government’s National Institute of Neurological Disorders and Stroke, nearly 3 million people worldwide — with almost a third in the United States — are living with multiple sclerosis (MS), a disabling neurological disease in which the body’s immune system mistakenly attacks nerves feeding information to the central nervous system (the brain and spinal cord). Although rarely fatal, MS can lead to long-term disabilities, and impair movement, muscle control, vision and cognition.
There currently is no cure for MS. However, findings from a new Johns Hopkins Medicine study provide strong support for a promising advance toward that goal: the ability to reverse — and in many cases, completely alleviate — MS-like symptoms in mice.
The study appears today in the journal Science Advances.
For an unknown reason in people with MS, some of the body’s first line of defense against foreign invaders — immune cells known as CD4+ T cells — fail to recognize that myelin (the fatty material surrounding and protecting nerve cells) is a normal part of the human system. If these wayward, or effector T cells, become dominant, they may provoke inflammation that damages or destroys the myelin sheath, which in turn, can severely disrupt or curtail transmission of nerve impulses from all parts of the body to the brain.
Scientists Design a Nanoparticle That May Improve mRNA Cancer Vaccines
Tests in mice with melanoma and colon cancer show tiny particle creates an “army” of immune cells that carry vaccine’s instructions, researchers say.

Johns Hopkins Medicine scientists say they have developed a nanoparticle — an extremely tiny biodegradable container — that has the potential to improve the delivery of messenger ribonucleic acid (mRNA)-based vaccines for infectious diseases such as COVID-19, and vaccines for treating non-infectious diseases including cancer.
Results of tests in mice, reported June 20 in the Proceedings of the National Academy of Sciences, show that the degradable, polymer-based nanoparticle carrying an mRNA-based vaccine, when injected into the bloodstream of mice, was able to travel to the spleen and activate certain cancer-fighting immune cells in a targeted way.
The researchers also found that mice with melanoma survived twice as long, and twice the number of mice with colorectal cancer survived long-term, following an injection of the Johns Hopkins-made nanoparticles compared with mice that received control treatments.
The 2023 Grantees of the Cohen Translational Engineering Fund Announced
This spring, two teams of faculty scientists affiliated with the Johns Hopkins School of Medicine, and the Department of Biomedical Engineering, received research grants through the Cohen Translational Engineering Fund.

The fund, made possible by a generous commitment from Sherry and Neil Cohen ’83, serves as a catalyst for translating cutting-edge research into practice by providing faculty with critical early funding. The grant is designed to help researchers move their work out of the laboratory and towards commercialization — the process includes developing patents, obtaining materials and supplies, and building prototypes.
“The pioneering work and level of innovation we have seen come out of the Whiting School of Engineering and Johns Hopkins overall is extremely impressive,” says Neil Cohen, founder and chairman of venture capital firm Emerald Development Managers. “We congratulate Dr. [Stephany] Tzeng, Dr. [Jordan] Green, Dr. [Nicholas] Durr and Mr. [Taylor] Bobrow on their achievements to date and look forward to following their progress. What delights us about the grants made by this fund is their ability to catalyze commercialization of important research while also helping the Whiting School of Engineering achieve its strategic goals of translating research into practice.”
Newly created protein a step toward preventing autoimmune disorders
Autoimmune diseases such as rheumatoid arthritis and multiple sclerosis happen when the immune system is inadvertently activated, mistakenly attacking the body’s tissues and organs. Though it is known that genetics play a role in the development of these disorders, prevention and treatment approaches also focus on external factors, such as nutrition and environment.
A team of Johns Hopkins engineers believes one answer to prevention and treatment lies inward, at the cellular level. They’ve designed a protein that activates and increases the number of special, regulatory T cells (called Tregs), which assist in preventing such disorders. Their results appear in Cell Reports.
“Tregs are critical for keeping our immune system in balance, and when they get out of whack, people can develop autoimmune diseases,” said Jamie Spangler, assistant professor in the departments of Chemical and Biomolecular Engineering and Biomedical Engineering and member of the research team. “(The study) showed that this molecule helps to prevent autoimmune diseases.”
“TREGS ARE CRITICAL FOR KEEPING OUR IMMUNE SYSTEM IN BALANCE, AND WHEN THEY GET OUT OF WHACK, PEOPLE CAN DEVELOP AUTOIMMUNE DISEASES.”
The molecule, which fuses the interleukin-2 cytokine and the anti-cytokine antibody F5111, promoted Treg activation and expansion and protected non-obese diabetic mice against autoimmune disease development to a statistically significant degree.
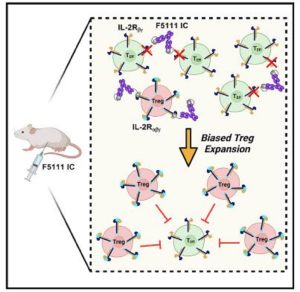
Image courtesy of Cell Reports
“The way in which it does this is by specifically targeting and expanding Tregs, which are used to suppress an immune response,” said the study’s lead author Derek VanDyke, PhD candidate in the Department of Chemical and Biomolecular Engineering. “In the case of autoimmune disease, your own immune system is essentially attacking itself, so these Tregs are used to suppress that attack.”
The authors say that because symptoms of autoimmune diseases are a result of the body’s defense system malfunctioning, suppressing this reaction could help in preventing the disease from manifesting. Since early detection and prevention is not always possible, however, future work will explore the possibility of using this approach to reverse active disease.
The research team included Johns Hopkins University School of Medicine’s Luke Tomasovic, Drew Pardoll, and Giorgio Raimondi and collaborators at the University of California, San Francisco; the Academy of Sciences of the Czech Republic; the University of Oxford; the University of Pennsylvania; and the University of California, Los Angeles.
Funding was provided by the National Institutes of Health; the Department of Defense; the Juvenile Diabetes Research Foundation; the Czech Science Foundation; the Institute of Biotechnology of the Czech Academy of Sciences; the EU Horizon project; ReSHAPE; and the Mark Foundation for Cancer Research.





